A Robotic Cloud Lab Partnership Between Strateos and Amgen
In a partnership between Strateos and Amgen, we have demonstrated the advantages of a programmatic cloud lab for supporting a multi-site drug discovery campaign.
The lab of the future is an integrated, collaborative platform leveraging high-throughput robotics, cloud computing, and bioinformatics to turn life sciences into an information technology. In a partnership between Strateos and Amgen, we have demonstrated the advantages of a programmatic cloud lab for supporting a multi-site drug discovery campaign. The primary objective was to transfer, from Amgen to the Strateos Robotic Cloud Lab, the responsibility for assay execution in support of weekly medicinal chemistry cycles while maintaining strict performance criteria. In this post we demonstrate the value and advantages of a programmatic cloud lab including reproducible experimental execution, flexible adaptation to process changes, and generation of rich datasets.
Cloud Lab Platform
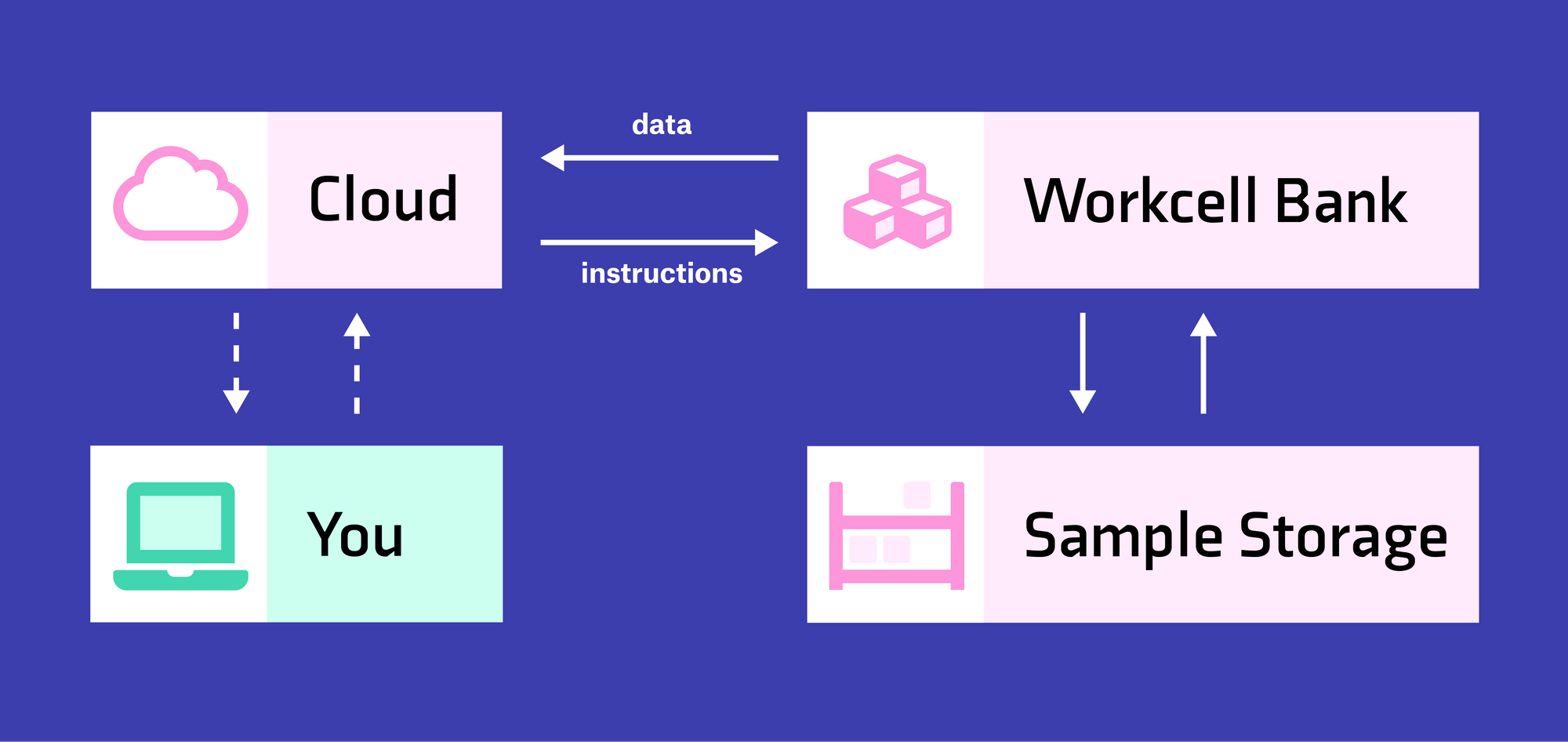
The Strateos Platform is powered by the Strateos Cloud and Automated Laboratory Environment (SCALE), a proprietary software stack that manages and orchestrates protocol code in the form of Autoprotocol, device drivers, and task scheduling for experimental execution (Figure 1). With Strateos’s cloud platform, users can easily design, run, and analyze experiments from anywhere in the world and have them fulfilled by automated robotic workcells (Figure 2).

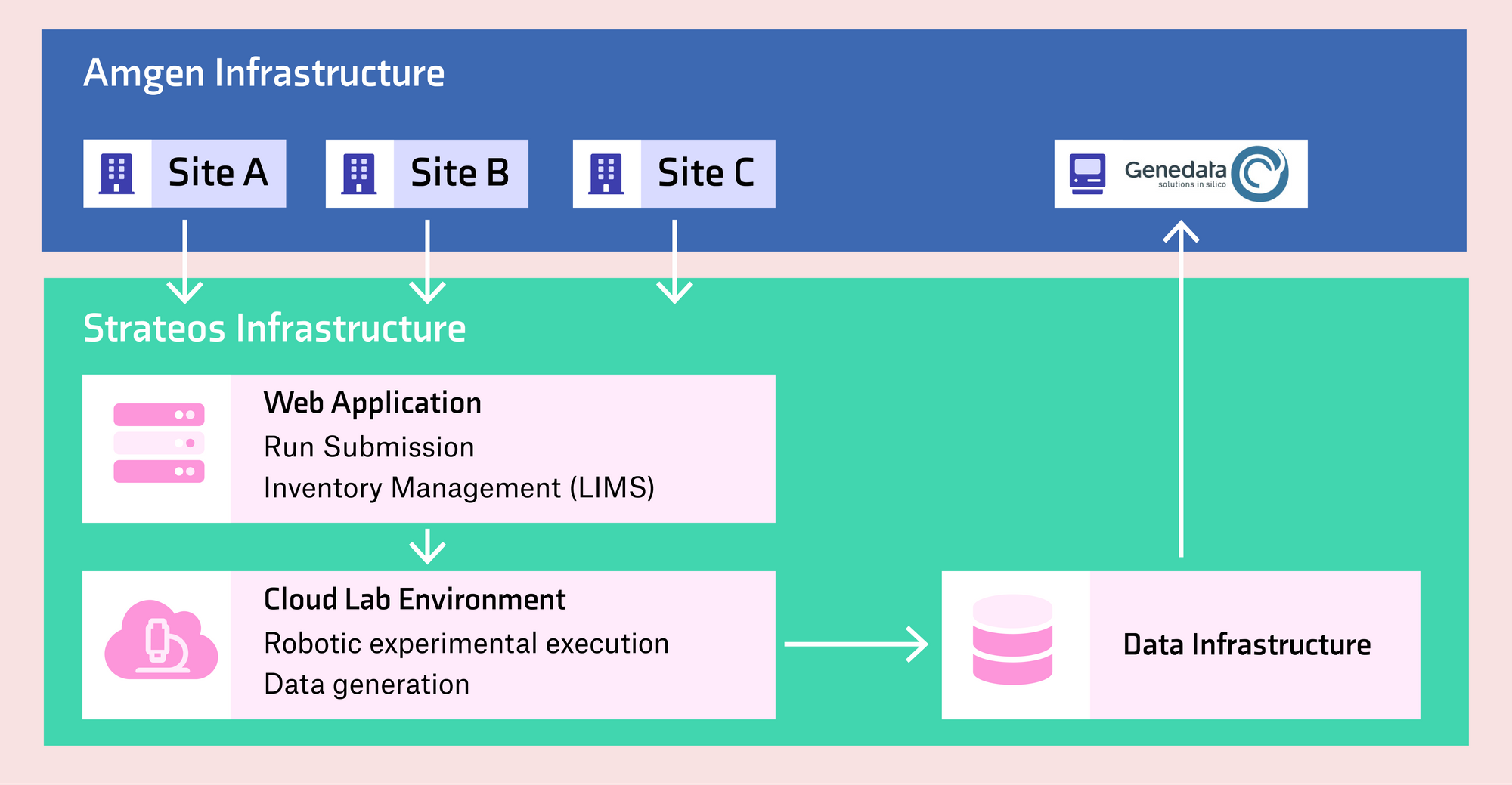
Amgen users across multiple departments submit runs for execution via the Strateos web application (WebApp). Upon experiment completion, raw and reformatted data are available via the WebApp and automatically pushed via an application programming interface (API) directly into an analysis pipeline (Genedata Screener) established at Amgen. The underlying Strateos infrastructure is coordinated by the WebApp for run and inventory management and SCALE as a lab operating system for automated execution and data generation. (Figure 3)
Autoprotocol is an open-source standard developed at Strateos for experimental specification in a way that is precise, unambiguous, and readable by both humans and machines. Strateos uses a Python library to convert natural-language English protocols into Autoprotocol for execution on Strateos's Robotics. Programmatic expression of protocols allows for several advantages including protocol versioning, explicit instructions, and execution transparency (Figure 4).

Results
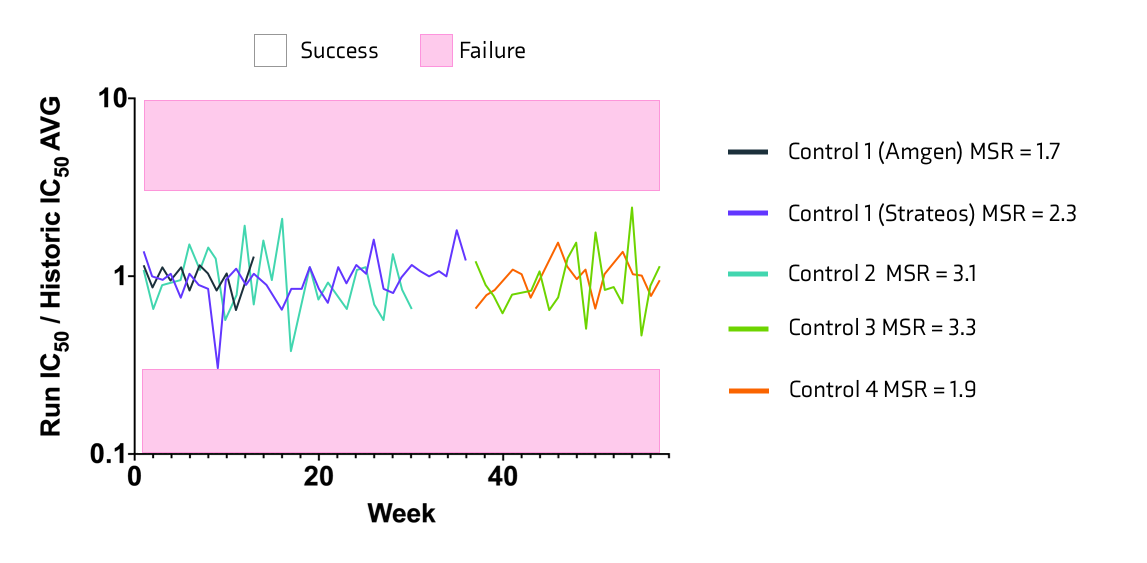
Four control compounds were utilized over the course of the project. Control compounds 1& 2 were replaced by more potent controls 3 & 4 approximately 30 weeks into the program. IC50 ratios were calculated for each of the reference standards including internally generated Amgen data for control 1 (Figure 5). Assay acceptance criteria was specified as a run having IC50 within 3-fold of the historic average. Minimal Significance Ratio (MSR) was calculated for each control compound (assay success criteria, MSR < 7.5).
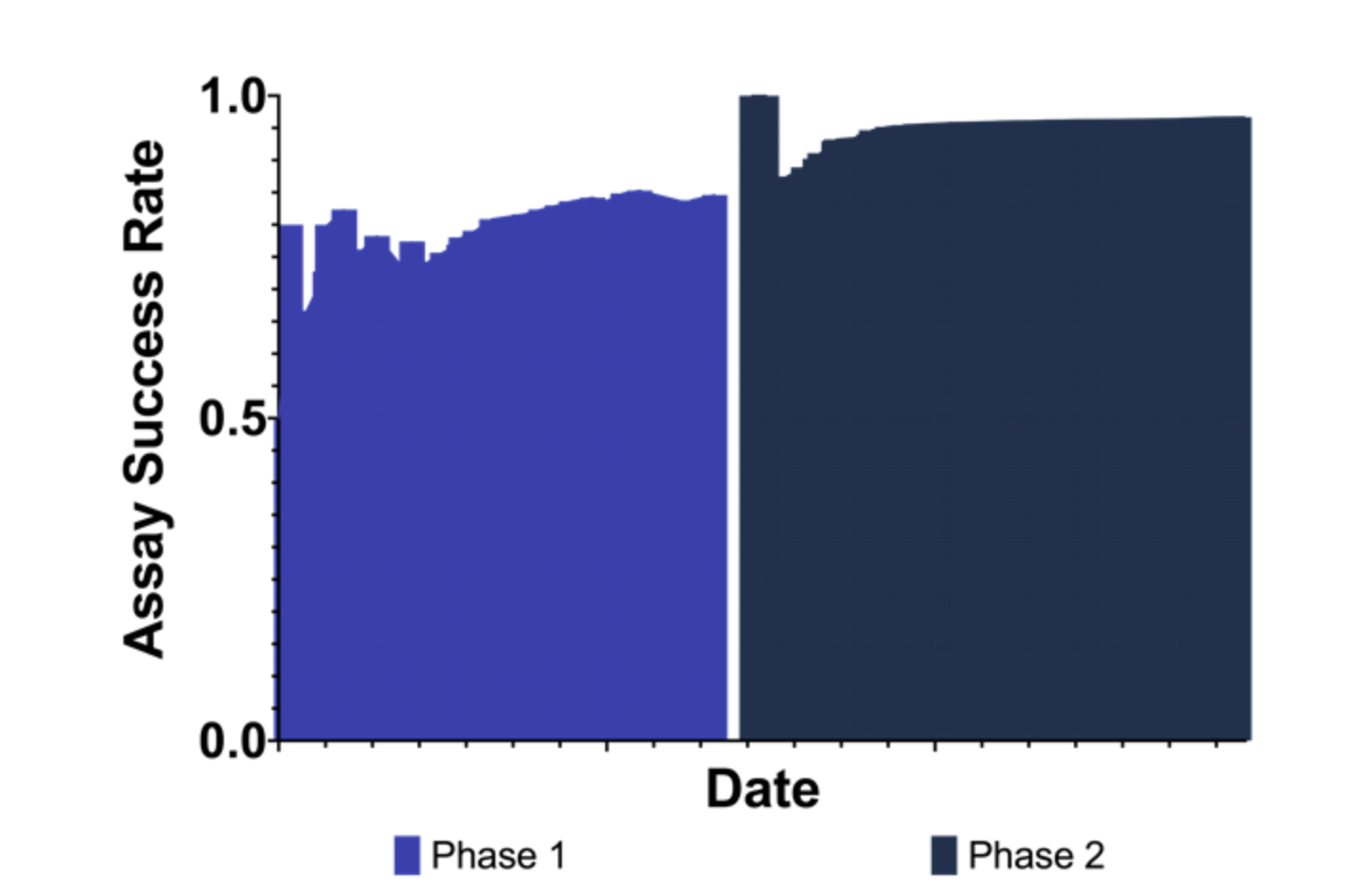
Cumulative assay first pass rate was calculated for each phase of the collaboration (Figure 6). Phase 1 displayed an overall first pass rate of 85%, whereas improvements to the assay led to a first pass rate of 97% for Phase 2.
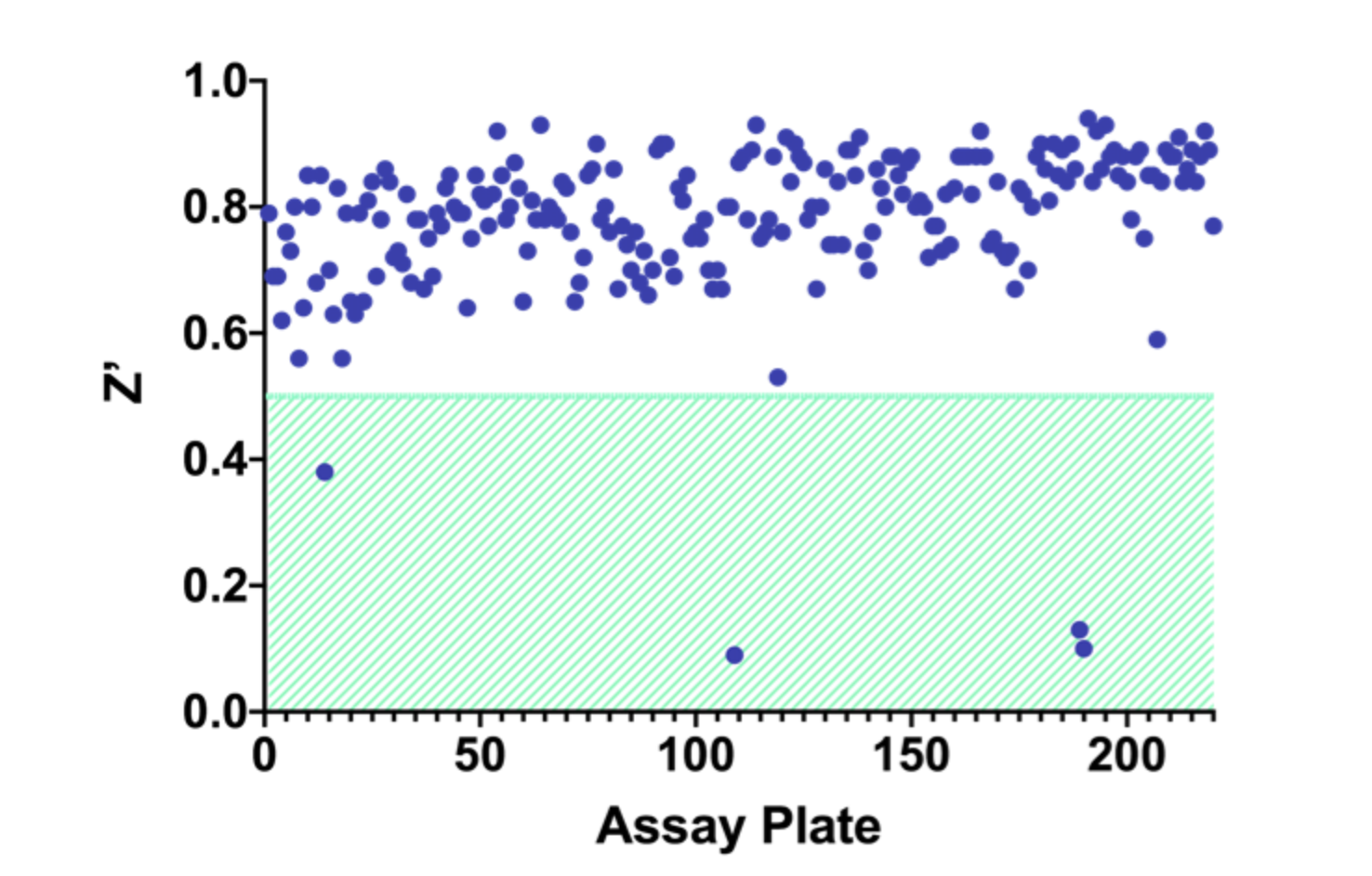
Assay Z-prime was calculated based on 32 control wells per assay plate (Figure 7). The assay met Z-prime success criteria in more than 98% of runs (assay success criteria, Z-prime > 0.5)
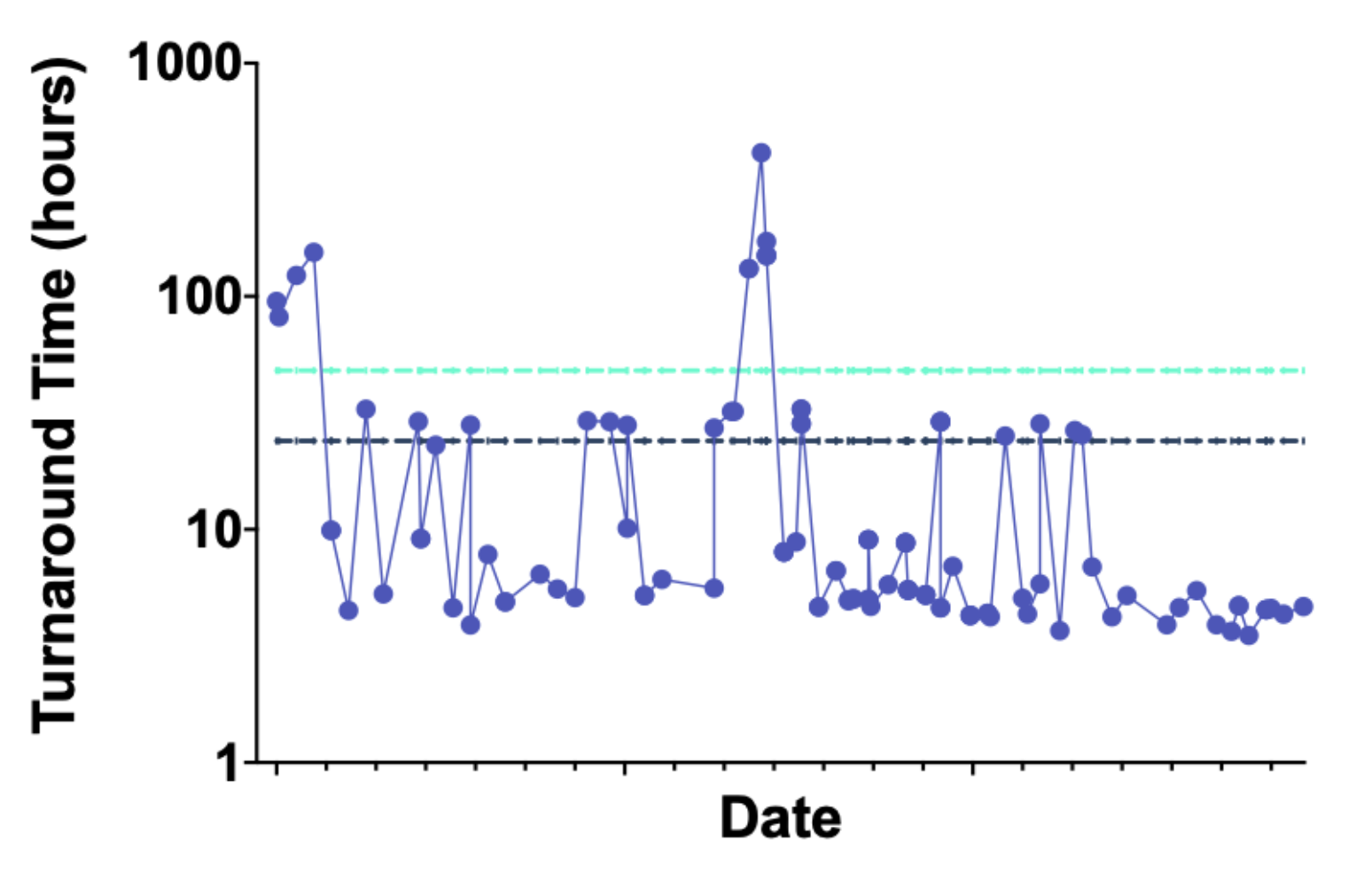
Turnaround time (TAT) was calculated by determining the time (hours) necessary from receipt of compound plate to delivering data meeting all assay success criteria (Figure 8). More than 90% of the compounds tested had valid data delivered within 32 hours of their arrival at Strateos.
A multi-year collaboration between Amgen and Strateos has successfully demonstrated the advantages of a programmatic cloud lab.
Conclusion
A multi-year collaboration between Amgen and Strateos has successfully demonstrated the advantages of a programmatic cloud lab. Amgen effectively utilized the Strateos platform for execution of assays either on a weekly basis (regular) or ad hoc (on demand) in support of medicinal chemistry efforts, thereby enabling discovery teams to focus their efforts on testing new hypotheses. The assays executed on the Strateos platform maintained a high level of performance with experimental reproducibility equivalent to internal Amgen runs with a median turnaround time of 5.7 hours and median assay Z-prime of 0.8.
Authors
Jeremy P. Hunt1, John Allen2, Yin He1, Vanessa M. Biggers1,Trissha R. Higa1, Noelle Javier3, Xi Liu1, Mei-Chu Lo3, Paolo Manzanillo3, Benjamin N. Miles1, Joseph G. McGivern3
1. Strateos, Inc. 3565 Haven Ave, Suite 3, Menlo Park, CA 94025, https://www.strateos.com/
2. Amgen, Inc. 1 Amgen Center Dr, Thousand Oaks, CA 91320, https://www.amgen.com/
3. Amgen, Inc. 1120 Veterans Blvd, South San Francisco, CA 94080, https://www.amgen.com/
Editor's note
The content of this blog post was originally published as a poster jointly presented by Amgen and Strateos at the Society for Lab Automation and Screening (SLAS) Conference 2020 in San Diego, CA.
